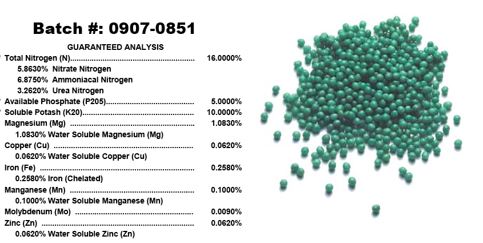
We all know that plants take up nitrogen in significant quantities, compared to some of the other essential nutrients. What most don’t know is that elemental nitrogen (N) is not what is taken up by plants. In fact, nitrogen can be taken up in only two forms, ammonium (NH4) and nitrate (NO3). Fertilizer labels will list the elements contained within, including the various types of nitrogen (Fig.1).
- Figure 1. Fertilizer labels will include the forms of nutrients included in the formulation.
What you should know about pH…..
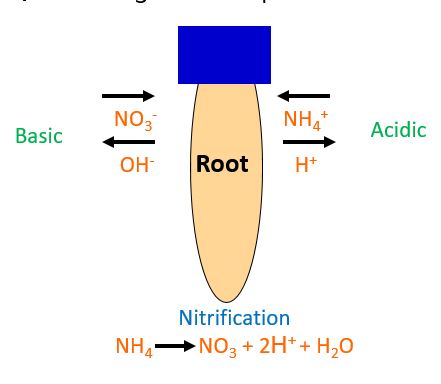
The definition of pH is the negative logarithmic of the hydrogen-ion concentration. What does this even mean? More simply put, the more H+ (hydrogen) ions, the more acidic, while the more OH– (hydroxide) the more basic. Always remember that the pH scale is logarithmic, which means each number on the scale is 10 times more acidic or basic than the next number on the scale. A pH of 6 is 10 times more acidic than a pH of 7. This is one reason changing pH dramatically is very difficult. Ammonium has a positive charge and nitrate has a negative charge. When a plant takes in a positive or negative charged ion, the roots will release the inversely charged ion. For example, when a root takes up ammonium, an H+ is released, while nitrate uptake releases OH–(Fig. 2). Ammonium nitrate is a common type of fertilizer due to the net neutral charge on soil pH. When looking to alter soil pH with a nitrogen source, there are options to pursue.
- Figure 2. Plant uptake of ammonium and nitrate, and the release of hydrogen and hydroxide. Illustration by Mike Mickelbart.
Impacts of soil pH….
Other than small pockets, the majority of the Midwest’s soils are alkaline (basic), with pH approaching the 7.5 to 8.0 range. Limestone bedrock dominates the region, which is the primary contributor to the elevated pH levels. Unfortunately many of our ornamental plants prefer a pH below 7, thus making some species susceptible to nutrient deficiencies. The two most common deficiency symptoms found in many ornamental plants involve iron (Fe) and manganese (Mn). The two essential nutrients are very sensitive to changes in pH, whereas these nutrients are not available for root uptake in alkaline soils.
How to develop a plan to lower pH….
There are a couple of methods to correcting the pH of the soil, which includes adding organic matter, sulfur containing products, and fertilizing using an acidifying nitrogen source (Fig. 3). As previously mentioned, if a lower pH is required, ammonium or urea-based fertilizers can aide in the reduction of pH.
- Figure 3. Acidity index of nitrogen sources.
More Information on Soil pH: https://www.purduelandscapereport.org//article/1658/
Fertilizing Woody Plants: https://www.extension.purdue.edu/extmedia/HO/HO-140-W.pdf
Collecting Soil Samples for Testing: https://www.extension.purdue.edu/extmedia/HO/HO-71-W.pdf
Soil pH and Organic Matter: http://landresources.montana.edu/nm/documents/NM8.pdf