It’s that time of year that roadways and sidewalks begin to be treated with salt. To prevent accidents, both vehicular and slips, salt is an invaluable tool that keeps people safe. For all of the benefits for humans, plants aren’t as appreciative. We have two great resources available that explains salt damage in great detail by Rosie Lerner and Janna Beckerman: Deicing salts helpful for people but not landscape plants and Salt Damage in Landscape Plants
- Figure 1. Deicing Salts Helpful for People but not Landscape Plants.
- Figure 2. Salt Damage to Landscape Plants Bulletin.
Calcium chloride (CaCl2) is sometimes used as the treatment for ice/snow, and is much safer to plants than sodium chloride (NaCl). The majority of the time NaCl is the primary de-icer due to the cost effectiveness compared to CaCl2, so the landscape can become sodic (high amounts of sodium). To remedy a salt (NaCl) buildup in the soil, there are few options available to improve conditions for plant growth.
One method to reduce the amount of sodium in soils is to irrigate deeply. It’s generally accepted that six inches of water will leach about 50% of salt accumulations in soil. This equates to approximately 372 gallons per 100 sq. ft. This method can take a significant amount of time and doesn’t leach all of the salt to acceptable levels.
The other, and most effective method, is to add Gypsum (calcium sulfate (CaSO4)) to the soil. Gypsum removes sodium by the interaction between the sulfate ions and sodium. The negatively charged sodium ions attract with the positively charged sodium ions, forming sodium sulfate, which is highly leachable from the soil. The calcium leftover from the reaction will bind to soil particles, which will provide aeration to the soil, thus increasing the leaching potential (Fig. 3). After gypsum is applied, irrigation is applied to leach out the sodium.
- Figure 3. Gypsum and irrigation leach out most of the toxic levels of sodium ions in a sodic soil. Figure courtesy of Rengasamy et.al., 2009. https://www.researchgate.net/publication/276417142_Diagnosis_and_management_of_sodicity_and_salinity_in_soil_and_water_in_the_Murray_Irrigation_region#fullTextFileContent
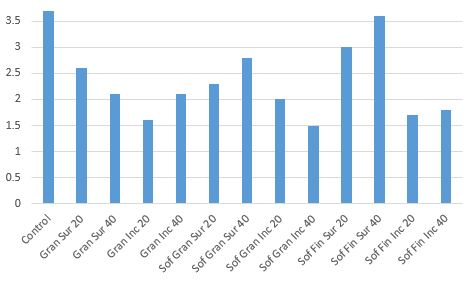
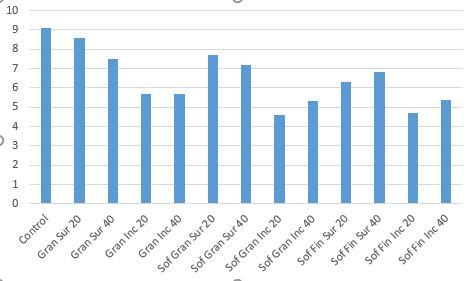
Dirr and Beiderman (1980) compared three separate gypsum products at two rates (20 and 40 pounds/100 sq. ft, respectively) and found that the two granular products improved plant appearance of cotoneaster and reduced electrical conductivity (amount of salts in the soil) compared to the control (Figs. 4 and 5).
- Figure 4. Visual ratings of cotoneaster after salt and gypsum treatments. 0=No damage, 5=Total death. Treatments: 20 and 40 pounds each of US Gypsum Granular, Sof’N’Soil Lawn and Garden Granular, and Sof-N’Soil Fine. Modified from Dirr and Biederman, 1980.
- Figure 5. Electrical conductivity of cotoneaster after salt and gypsum treatments. 0=No damage, 5=Total death. Treatments: 20 and 40 pounds each of US Gypsum Granular, Sof’N’Soil Lawn and Garden Granular, and Sof-N’Soil Fine. Modified from Dirr and Biederman, 1980.
Prevention is always the best medicine, so limit the amount of salt around landscapes if possible, select salt-tolerant plants, and use calcium chloride instead of sodium chloride. When you are faced with sodic soils from salt buildup, a combination of gypsum and heavy irrigation should be used to reduce the amount of sodium around plants. It’s best to perform this technique prior to bud break in the spring so that deciduous plants aren’t yet up taking water, thus preventing plant damage.
Literature Cited:
Dirr, M. and Beiderman, J. 1980. Amerlioration of salt damage to cotoneaster by gypsum. Journal of Arboriculture. 6(4).
Rengasamy, P., North, S., and Smith, A. 2009. Diagnosis and management of sodicity and salinity in soil and water in the Murray Irrigation region. The University of Adelaide, SA. 98 pgs. https://www.researchgate.net/publication/276417142_Diagnosis_and_management_of_sodicity_and_salinity_in_soil_and_water_in_the_Murray_Irrigation_region#fullTextFileContent. Last accessed 12/9/2020.